11
Case
Age: 55-year-old black man
Reason for referral to ophthalmology: Glaucoma
Past medical history: None
Past ocular history: None
Medications: None
Habits: Non-smoker and does not drink alcohol
Family history: Father with glaucoma treated with topical medications
HPI: He went to see an optometrist for a routine examination and was found to have optic nerve cupping in both eyes. His intraocular pressure was 20 OD and 21 OS and his central corneal thickness was 550 µm OD and 546µm OS. An ophthalmology consultation was requested for further management.
Ophthalmological examination:
Blood pressure: 118/80, heart rate 74
Visual acuity: 20/20 OD, 20/20 OS
Pupils are equal sizes and reactive to light, there is no RAPD
Color vision: 14/14 OD and 14/14 OS correct Ishihara plates
Ocular motility and alignment are normal
Slit lamp examination is normal
Neurological examination is normal
1. Which of the following is true regarding intraocular pressure (IOP) and glaucoma?
- All patients with glaucoma have elevated IOP at presentation
- IOP can vary by 10 mm Hg or more over a period of 24 hours in patients with glaucoma
- IOP is an adequate screening test for glaucoma
- The mean IOP in the population is 21 mm Hg
1. Which of the following is true regarding intraocular pressure (IOP) and glaucoma? 2. IOP can vary by 10 mm Hg or more over a period of 24 hours in patients with glaucoma
Elevated IOP is an important risk factor for primary open angle glaucoma. The mean IOP in the population is 15.5 mm Hg with a standard deviation of 2.6. This has led to the “normal” range of IOP of 10-21, which is 2 standard deviations from the mean. Many population-based studies studies have found that as many as 30 to 50% of individuals with glaucoma and visual field loss have initial screening IOPs less than 22 mm Hg. Intraocular pressure can vary considerably, by more 10 mm Hg in 24 hours, in patients with glaucoma. Some patients with glaucoma have IOPs that are consistently within the “normal” range, which is termed normal-tension glaucoma. Patients with optic nerve cupping and normal IOPs often pose the most difficulty in differentiating glaucomatous from non-glaucomatous optic neuropathies.
Clinical Pearl
Intraocular pressure within the normal range does not rule out glaucoma.
2. What visual acuity is expected in a patient with moderate glaucoma and no other ocular pathology?
- Normal visual acuity
- Mildly reduced visual acuity
- Moderately reduced visual acuity
- Severely reduced visual acuity
2. What visual acuity is expected in a patient with moderate glaucoma and no other ocular pathology? 1. Normal visual acuity
Patients with glaucoma, even in advanced stages, usually have preserved visual acuity and colour vision since glaucoma tends to spare the papillomacular bundle. This is a useful clue in differentiating glaucomatous and non-glaucomatous optic neuropathy. The latter tends to involve the papillomacular bundle early, causing reduced central vision.
Clinical Pearl
Reduced visual acuity (especially without total cupping) is concerning for non-glaucomatous optic neuropathy.
3. What clues from the slit lamp examination would favour glaucoma over a non-glaucomatous optic neuropathy?
- Pseudoexfoliative material on the lens capsule
- Krukenberg spindle
- Peripheral anterior synechiae
- Narrow angles
- All of the above
3. What clues from the slit lamp examination would favour glaucoma over a non-glaucomatous optic neuropathy? 5. All of the above
When assessing a patient with optic nerve cupping, signs of secondary glaucoma suggest a glaucomatous rather than a non-glaucomatous optic neuropathy. These signs include pseudoexfoliative material on the pupillary border or lens capsule (pseudoexfoliation syndrome), Krukenberg spindle, heavily pigmented trabecular meshwork, and iris transillumination defects (pigment dispersion syndrome), posterior or peripheral anterior synechiae and anterior chamber cells (uveitic glaucoma), and occludable or narrow angles (narrow angle glaucoma). Usually, these signs are associated with an elevated intraocular pressure.
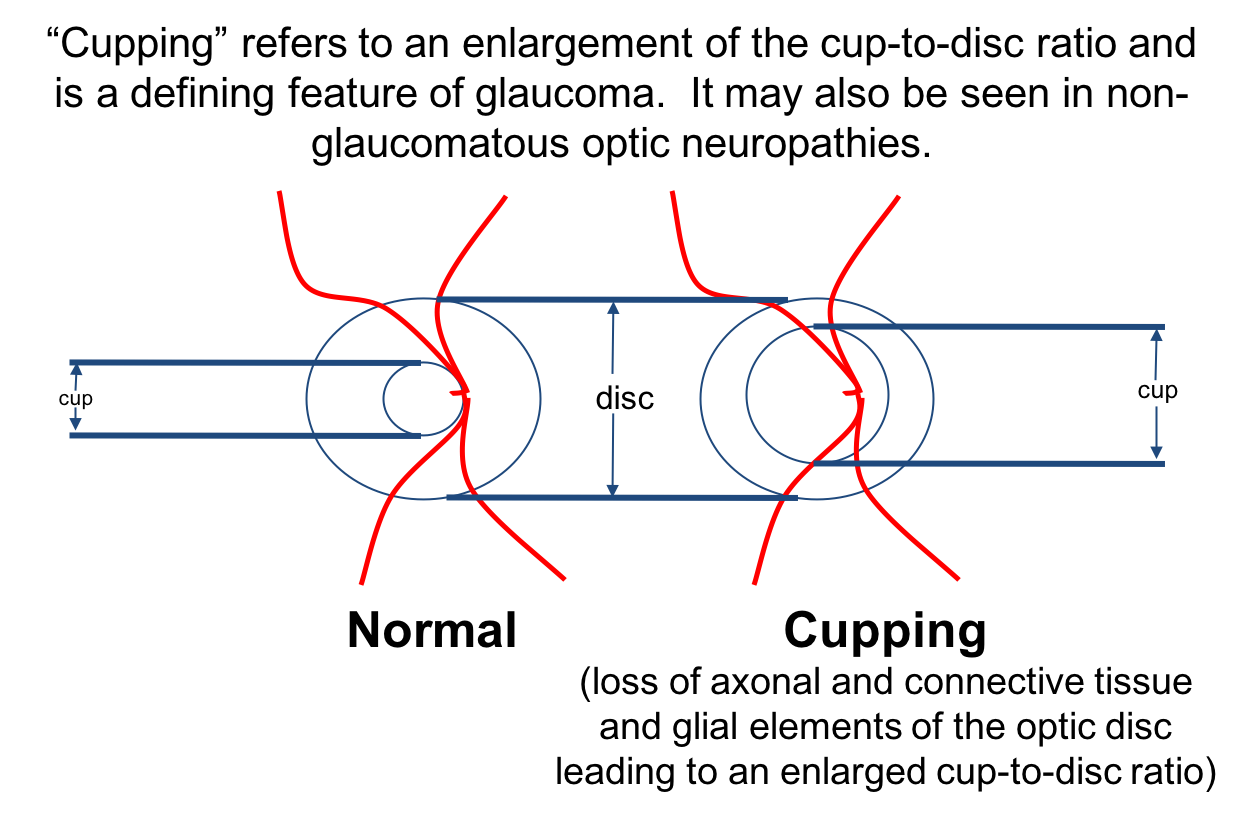
4. Cupping occurs when there is:
- Loss of the retinal nerve fiber layer only
- Loss of the retinal nerve fiber layer and connective tissue and glial elements of the optic disc
- Loss of the retinal nerve fiber layer and inner nuclear layer
- Choroidal thinning
4. Cupping occurs when there is: 2. Loss of retinal nerve fiber layer and connective tissue and glial elements of the optic disc
When there is loss of axons of the optic disc, but not the connective tissue and glial elements, the optic nerve appears pale and usually does not have significant cupping. When both the axonal and supporting connective tissue are lost, the nerve appears cupped as demonstrated below.
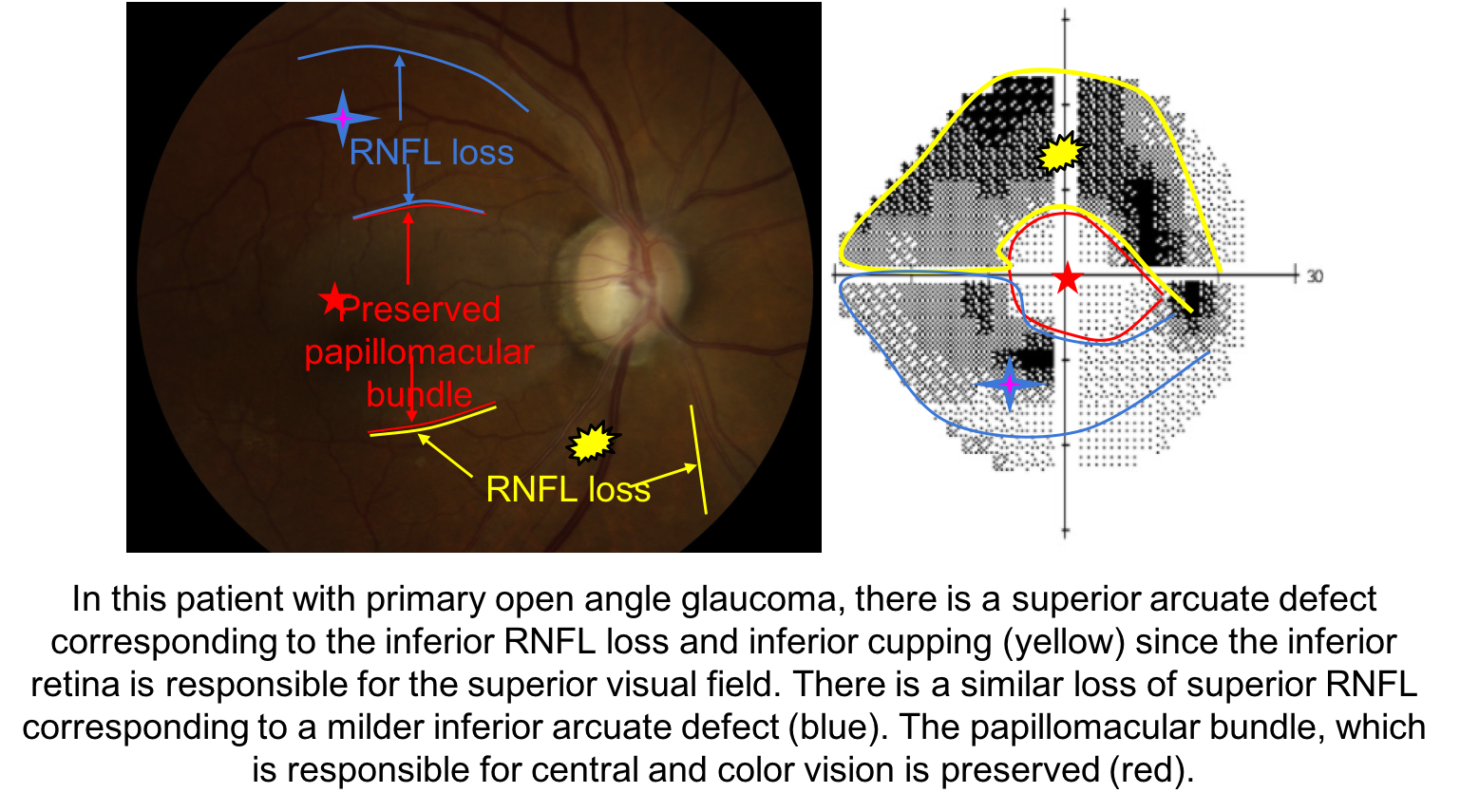
5. What visual field defect is expected in this patient with glaucoma?
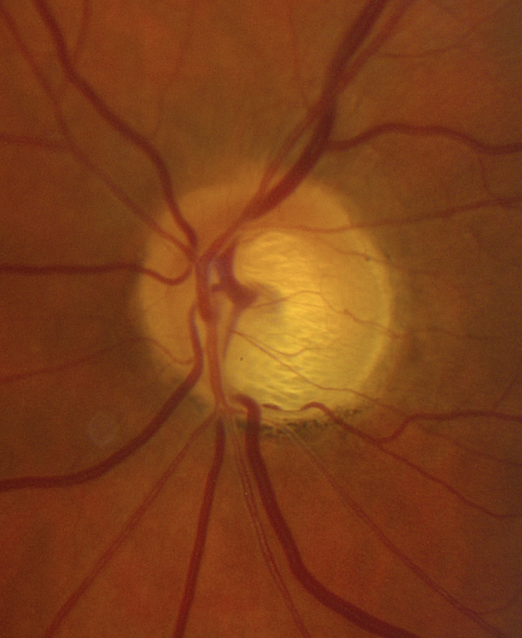
- Severely constriction
- Superior arcuate
- Inferior arcuate
- Central scotoma
5. What visual field defect is expected in this patient with glaucoma? 2. Superior arcuate
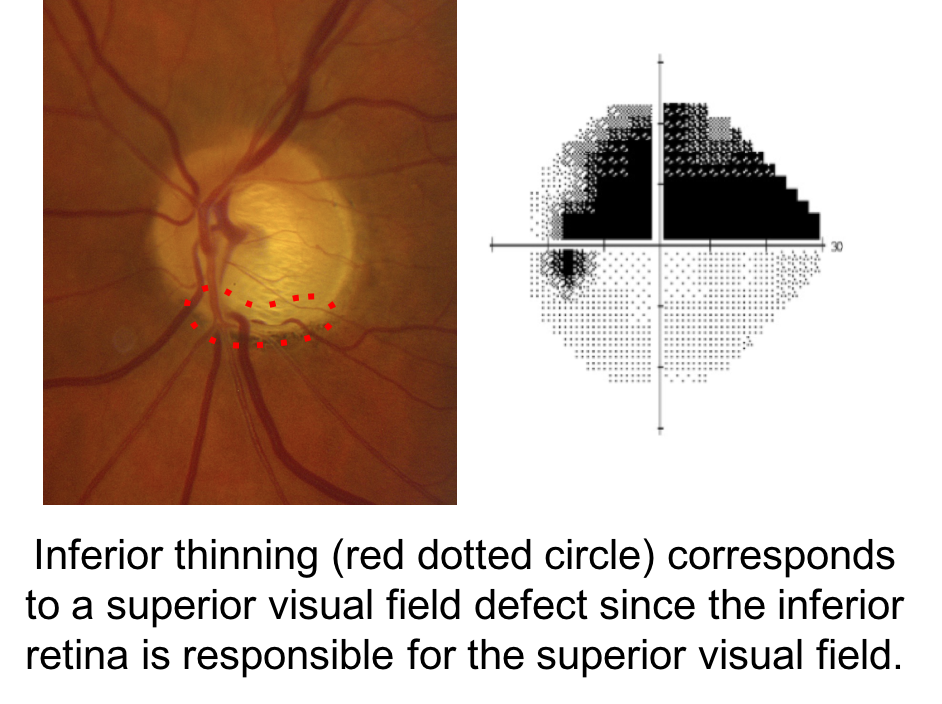
When assessing a patient for possible glaucoma, it is important to ensure that the optic nerve changes match the visual field defect. This patient has prominent loss of the neuroretinal rim inferiorly, which corresponds to a superior altitudinal visual field defect. If the optic nerve changes do not match the visual field or the visual loss is out of proportion to the optic nerve changes, than a non-glaucomatous optic neuropathy should be suspected.
6. Which of the following optic nerves most likely represents glaucoma?
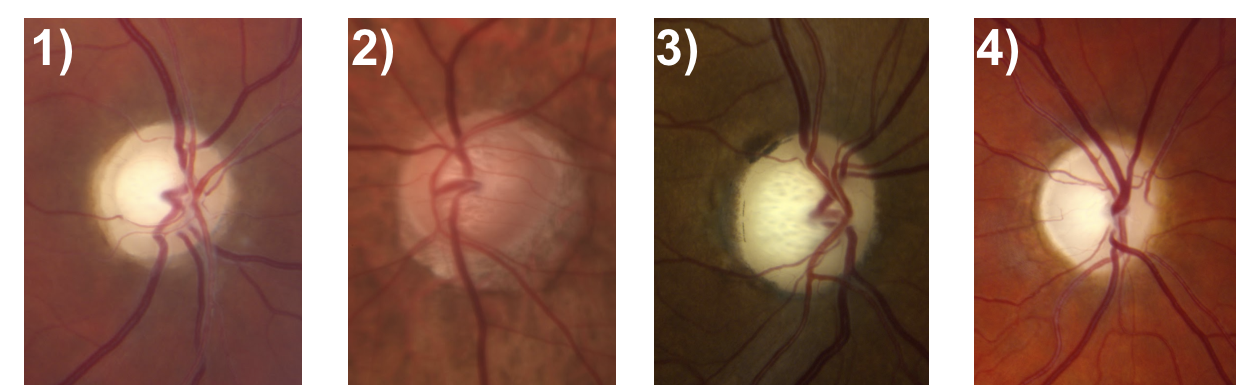
6. Which of the following optic nerves most likely represents glaucoma? 2.
The optic nerve depicted in (2) has an enlarged cup-to-disc ratio, but the remaining neuroretinal rim is a normal, pink color. The other optic nerves have pallor of the neuroretinal rim out of proportion to the cupping, which is characteristic of non-glaucomatous optic neuropathies. Other features of a glaucomatous optic nerve are listed in the box below.
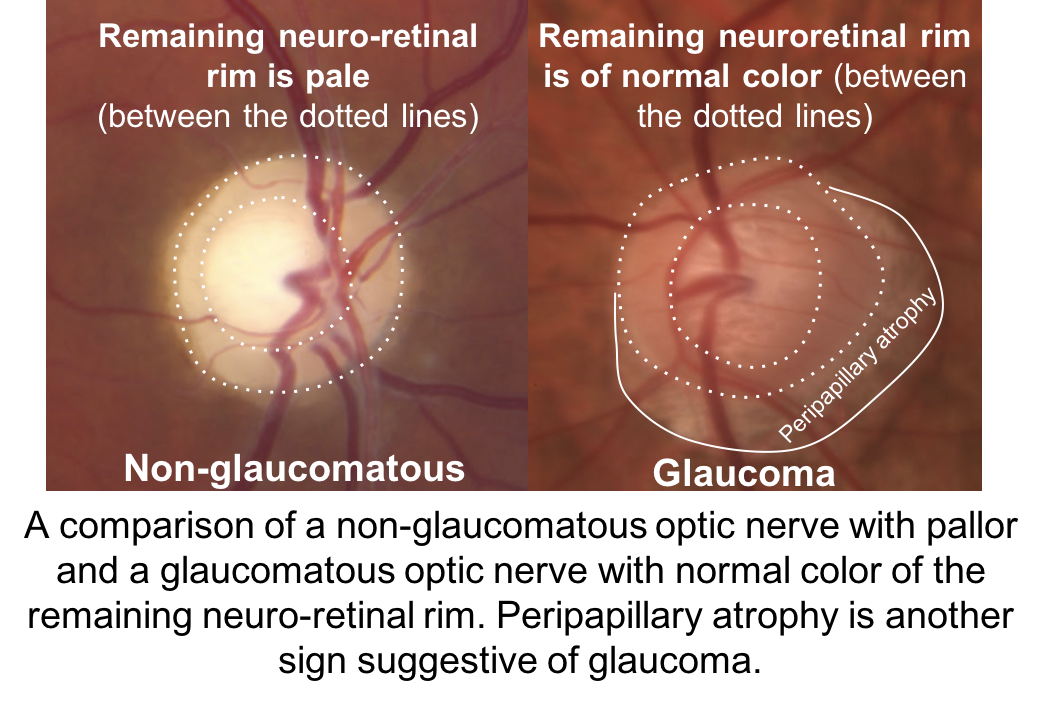
It is not always possible to differentiate glaucomatous and non-glaucomatous optic neuropathies just by assessing the optic nerve. Optic nerve pallor is a subjective sign that is not always obvious and there may be little remaining neuroretinal rim to adequately assess this feature in patients with advanced disease. Some individuals may have anolmalous optic nerves and cupping cannot be reliably assessed.
Clinical Pearl
Clinical features of a glaucomatous optic nerve include:
- Vertical elongation of the cup
- Focal elongation of the cup (notching)
- Normal color of the neuroretinal rim
- Excavation of the cup
- Peripapillary atrophy
- Disc hemorrhage (seen more frequently in normal tension glaucoma)
- Total cupping
- Exposed lamina cribrosa
- Baring of circumlinear vessels
7. Which optic neuropathies may demonstrate optic nerve cupping (enlarged cup-to-disc ratio)?
- Compressive optic neuropathy
- Hereditary optic neuropathy (Eg. Leber’s Hereditary Optic Neuropathy)
- Arteritic anterior ischemic optic neuropathy
- Traumatic optic neuropathy
- All of the above
7. Which optic neuropathies may demonstrate optic nerve cupping (enlarged cup-to-disc ratio)? 4. All of the above
Compression of the anterior visual pathways from tumors or aneurysms, hereditary optic neuropathies, arteritic ischemic optic neuropathies, and traumatic optic neuropathies may all cause cupping. Shown below is a patient with a right compressive optic neuropathy from an internal carotid artery aneurysm. Notice that there is cupping and pallor in the right eye, which is more obvious in comparison to the normal, left eye.
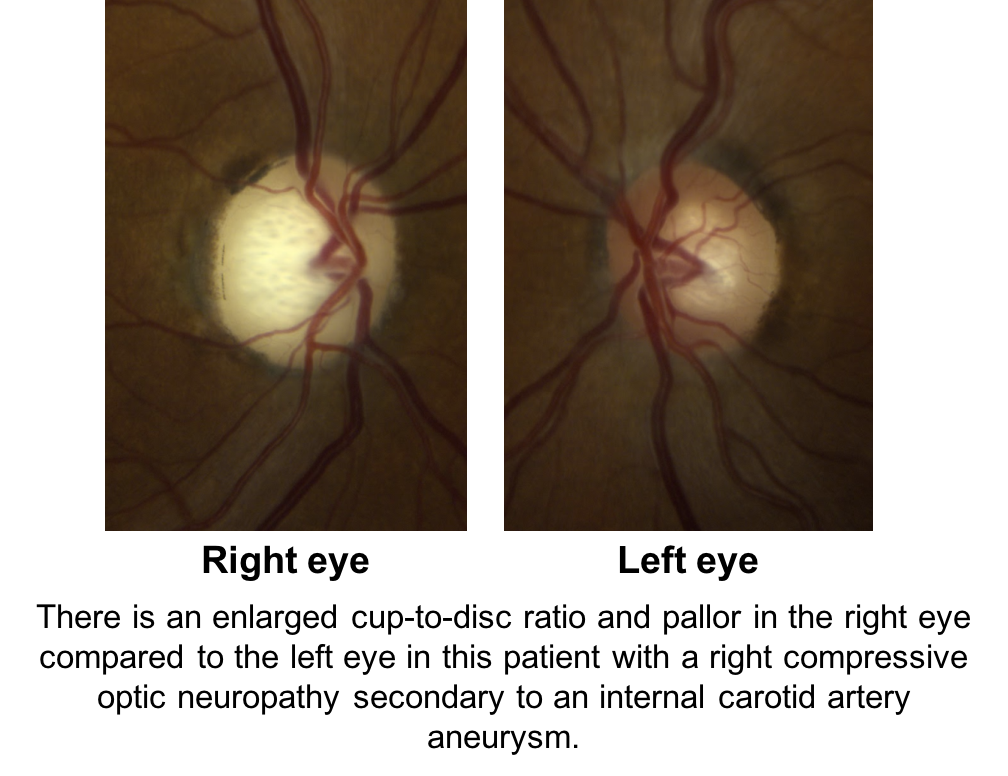
8. What other feature from the history or physical exam would be concerning for a non-glaucomatous optic neuropathy?
- Rapid progression of vision loss
- Intolerance of topical glaucoma medications
- Family history of glaucoma
- Previous use of longterm topical steroids
8. What other feature from the history or physical exam would be concerning for a non-glaucomatous optic neuropathy? 1. Rapid progression of vision loss
Although there are a number of factors that determine progression, glaucoma is typically a slowly progressive disease. A patient with rapidly progressing vision loss without an adequate explanation (such as uncontrolled IOP) should be assessed for non-glaucomatous etiologies.
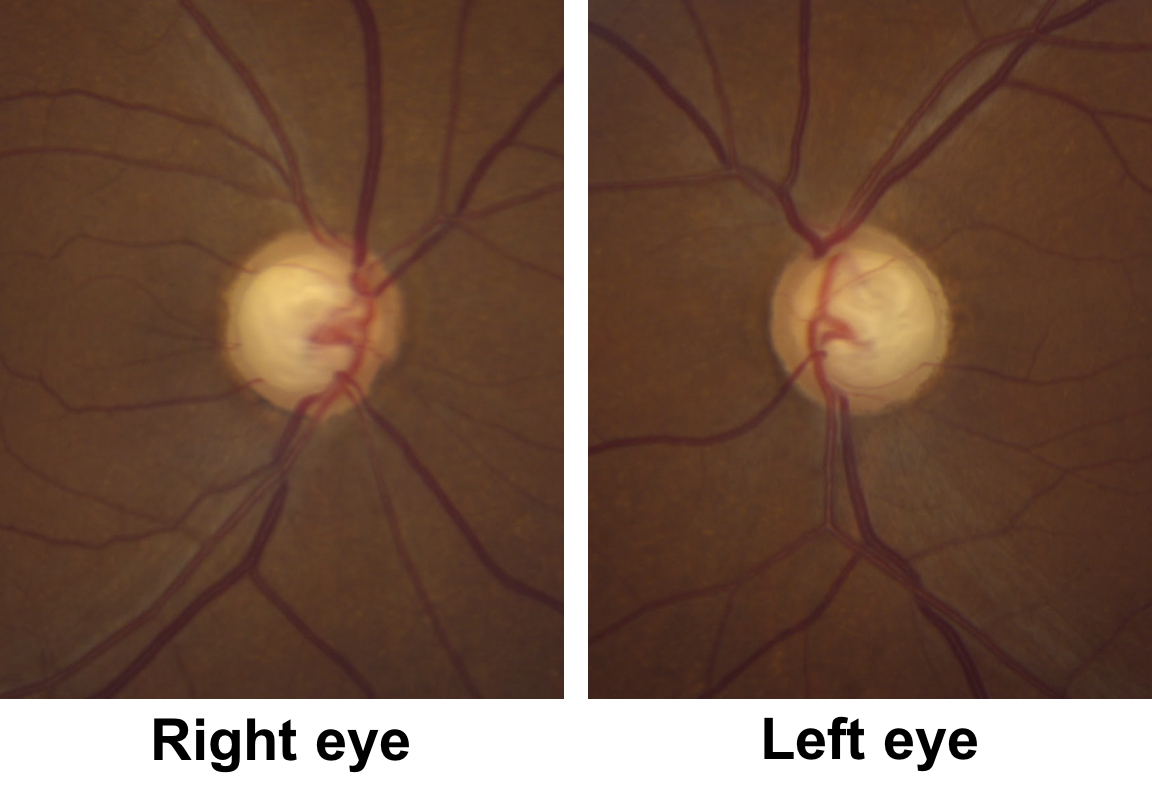
9. Returning to the original case, what do the optic nerves (shown below) demonstrate?

- Bilateral optic nerve pits
- Cupping with pallor of the remaining neuroretinal rim
- Optic disc edema
- Optic disc hemorrhages
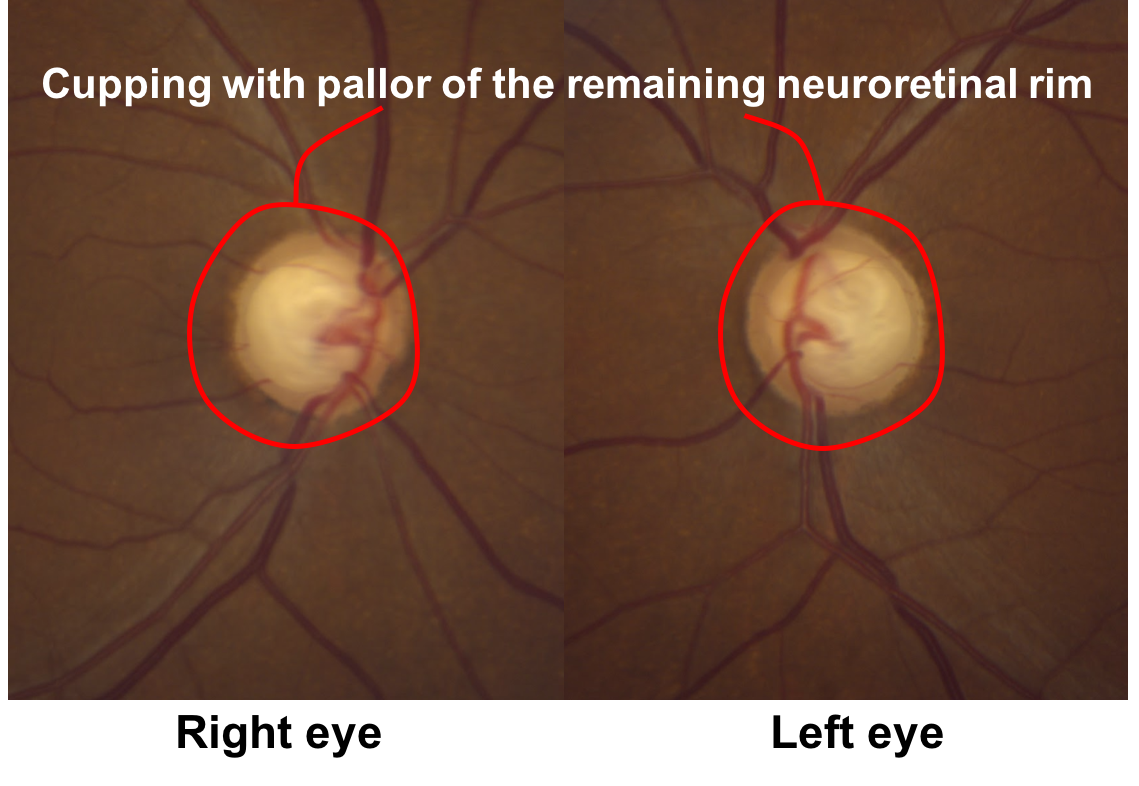
9. Returning to the original case, what do the optic nerves demonstrate? 2. Cupping with pallor of the remaining neuroretinal rim
This patient has obvious cupping with a cup-to-disc ratio of approximately 0.9. There is also pallor of the remaining neuroretinal rim, although this is not as obvious as the non-glaucomatous optic nerves shown in question 6.
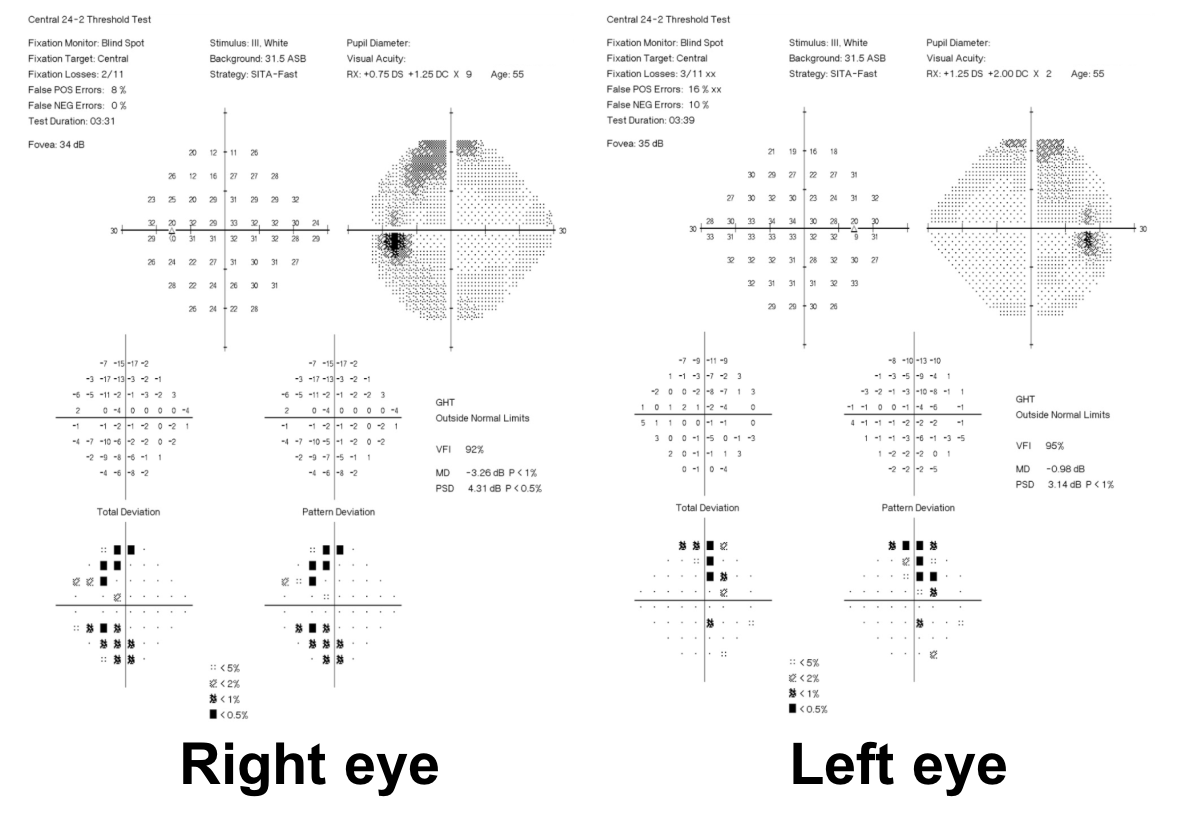
10. What visual field defect does this patient have?
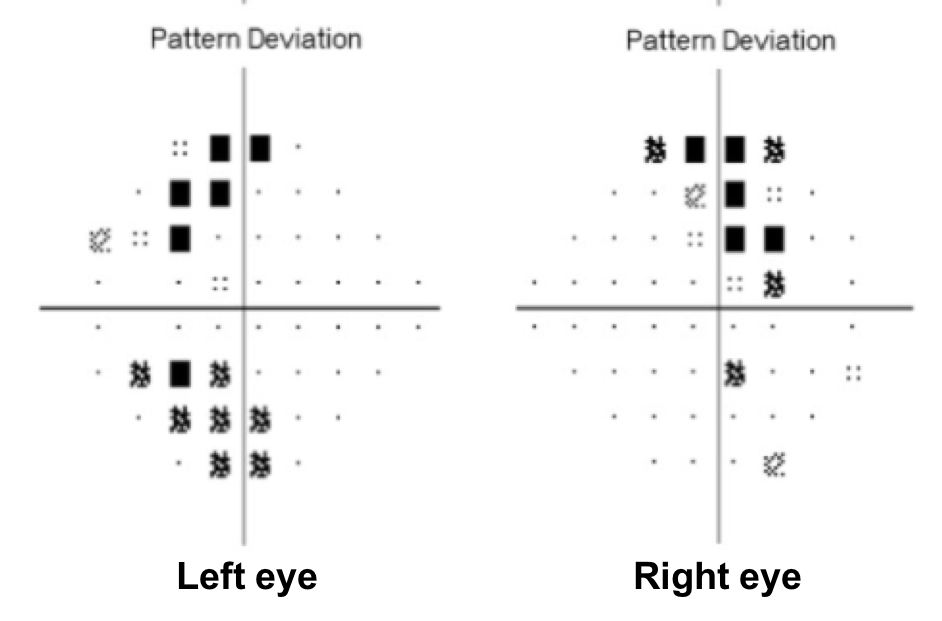
- Non-specific changes
- Arcuate defects
- Bitemporal hemianopia
- Cecocentral scotomas
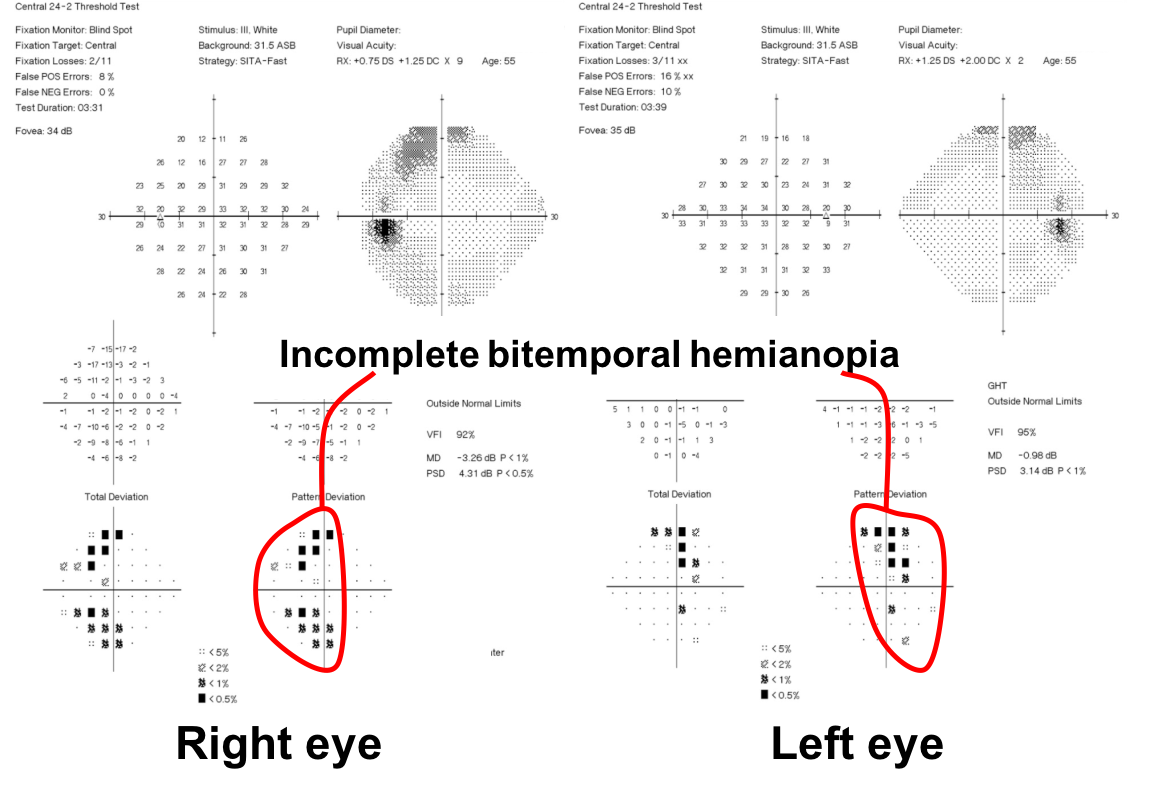
10. What visual field defect does this patient have? 3. Bitemporal hemianopia
This patient’s visual field appears to respect the vertical meridian and is best appreciated on the pattern deviation. Although there are some depressed points in the nasal visual field, there is a clear predominance in the temporal hemifield in both eyes. This is highly suggestive of a bitemporal hemianopia.
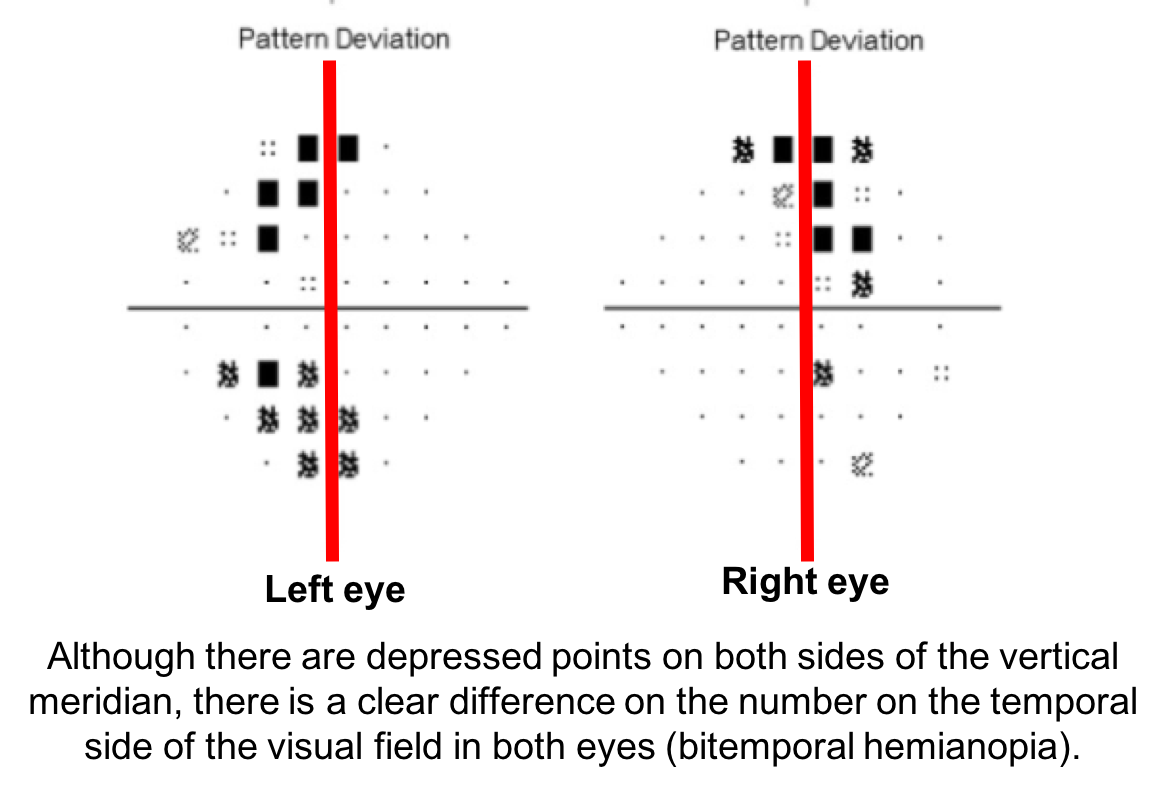
11. What is the next best course of action for this patient?
- Initiation of topical glaucoma medication
- Laser trabeculoplasty
- MRI of the brain and orbits with contrast
- CT scan of the head without contrast
11. What is the next best course of action for this patient? 3. MRI of the brain and orbits with contrast
The patient has a visual field defect that respects the vertical meridian and optic nerve cupping with pallor of the neuroretinal rim. This is concerning for a compressive lesion of the optic chiasm and an MRI of the brain and orbits is the best way to assess this. The MRI revealed a large pituitary adenoma (shown below).
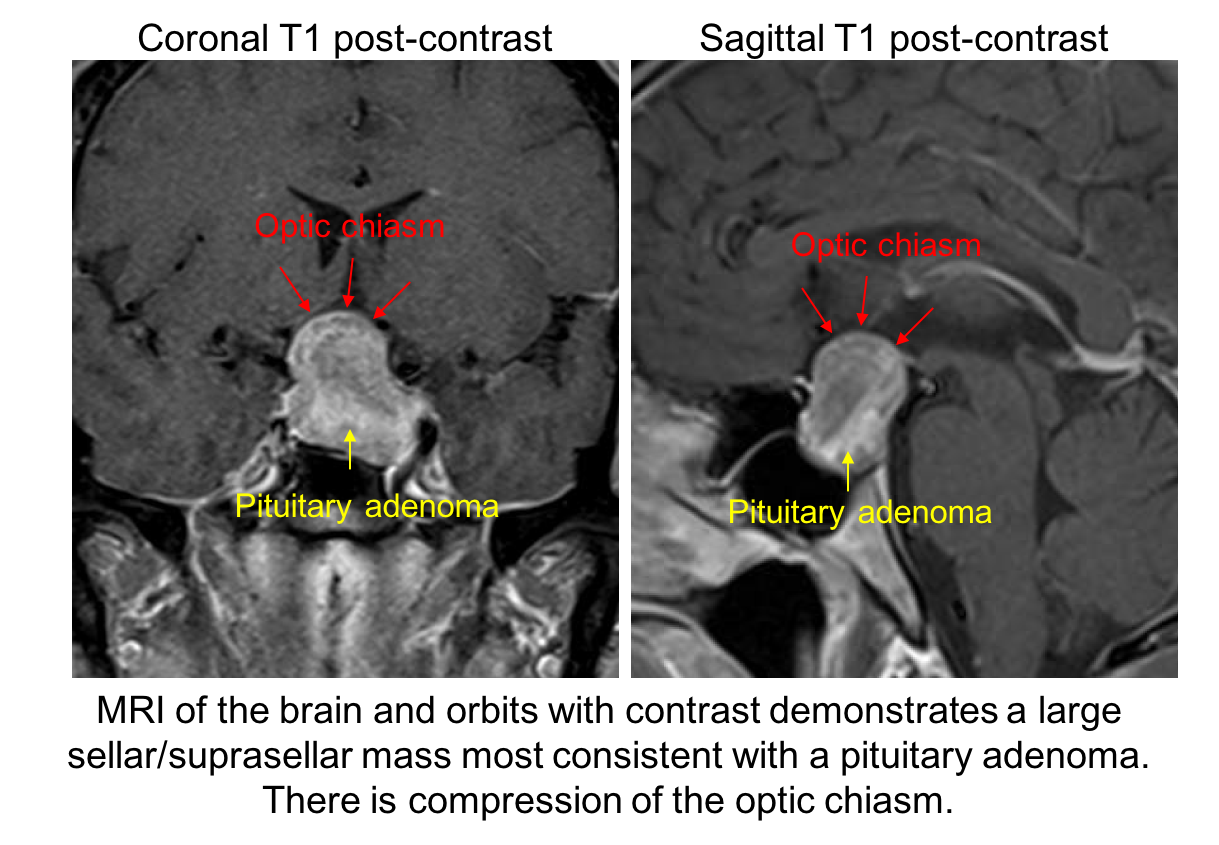
12. Which of the following is the best predictor of visual recovery after surgical removal of the pituitary adenoma?
- Pre-operative visual acuity
- Pre-operative retinal nerve fiber layer (RNFL) and ganglion cell complex thickness measured by optical coherence tomography (OCT)
- Age
- Duration of symptoms
12. Which of the following is the best predictor of visual recovery after surgical removal of the pituitary adenoma? 2. Pre-operative RNFL and ganglion cell complex thickness measured by OCT
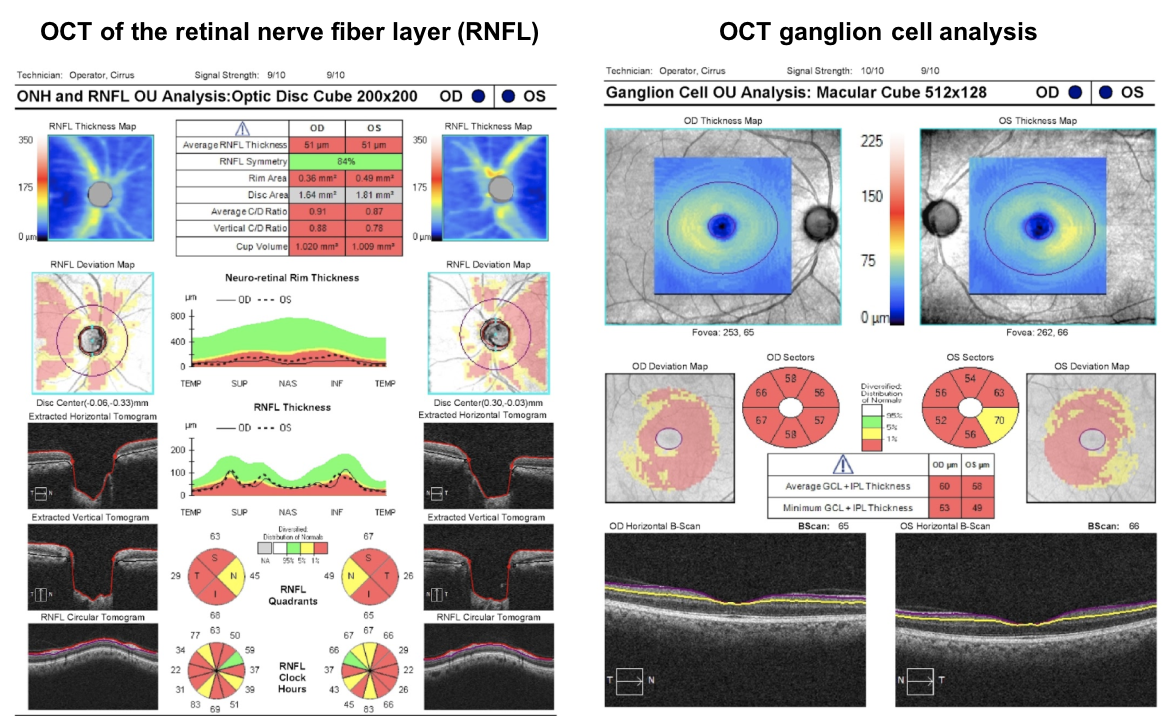
Most visual recovery after resection of a pituitary adenoma occurs in the first 6-10 weeks, although there is continued improvement up to one year after surgery. Most patients will show improvement after surgery, but those with a normal OCT RNFL thickness are more likely to return to a near normal visual field. OCT of the RNFL and macular ganglion cell complex are recommended by the Congress of Neurological Surgeons as a prognostic tool in patients with visual loss from pituitary tumors.
This patient’s OCT of the RNFL and ganglion cell complex are shown below. Since he has a very low RNFL thickness (51 um in both eyes), there is a lower chance of visual recovery compared to a patient with a normal RNFL thickness.
13. What else should be included in the pre-operative evaluation of this patient?
- Extended cardiac monitoring
- Genetic counselling
- Endocrine evaluation for hypopituitarism
- MRI of the spine
13. What else should be included in the pre-operative evaluation of this patient? 3. Endocrine evaluation for hypopituitarism
Endocrine evaluation to assess for hypopituitarism is recommended in all patients with pituitary adenomas. There is a high prevalence of hypopituitarism in patients with nonfunctioning pituitary adenomas and all anterior pituitary axes should be assessed. The cutoff values to initiate thyroid and adrenal replacement may be different in a patient with panhypopituitarism compared to isolated deficiencies and is best evaluated by an endocrinologist with expertise in this area.
Case Summary
On a routine examination, he was found to have bilateral optic nerve cupping and his visual field showed an incomplete, bitemporal hemianopia. MRI of the brain showed a large sellar/suprasellar mass consistent with a pituitary adenoma. He had a transphenoidal resection of the tumor and had improvement in his visual field, although it did not completely return to normal.
Further reading:
-
Ambati BK, Rizzo III JF. Nonglaucomatous cupping of the optic disc. Int Ophthalmol Clin 2001;41(1):139-49. https://www.ncbi.nlm.nih.gov/pubmed/11198141
-
Bianchi-Marzoli S, Rizzo III JF, Brancato R, Lessell S. Quantitative analysis of optic disc cupping in compressive optic neuropathy. Ophthalmology 1995;102:36-440. https://www.ncbi.nlm.nih.gov/pubmed/7891982
-
Danesh-Meyer HV, Wong A, Papchenko T, et al. Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci 2015;22:1098-1104. https://www.ncbi.nlm.nih.gov/pubmed/25891894
-
Newman SA, Turbin RE, Bodach ME, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Pretreatment Ophthalmology Evaluation in Patients With Suspected Nonfunctioning Pituitary Adenomas. Neurosurgery 2016;79:E530-532. https://www.cns.org/guidelines/guidelines-management-patients-non-functioning-pituitary-adenomas/Chapter_4